

(Written by Zhong Shuangxia / Translated by Chen Zhiying)Under the situation of the spreading COVID-19, the vaccine has become the best weapon, injecting great impetus to global epidemic prevention. As more and more vaccines appear around the globe, China’s vaccines are crossing the sea, helping to fight the epidemic globally. The vaccines going global have been recognized by the world, showing great potential.
China’s vaccine brings “timely rain” to the fight against the epidemic around the globe
The severe epidemic is pushing the research and development of global vaccines at a speed of record high. At present, the vaccines approved for marketing in the world mainly include America’s Pfizer, Britain’s AstraZeneca, China’s Sinopharm and Sinovac, Russia’s “Sputnik V”, and India’s Covaxin, etc. They can be mainly categorized into the inactivated vaccine, virus vector vaccine, recombinant protein vaccine and nucleic acid (DNA and RNA) vaccine.
Among all the global vaccines, China’s vaccine is particularly popular. Many countries have placed orders and signed procurement contracts with China, including more than half of ASEAN countries.
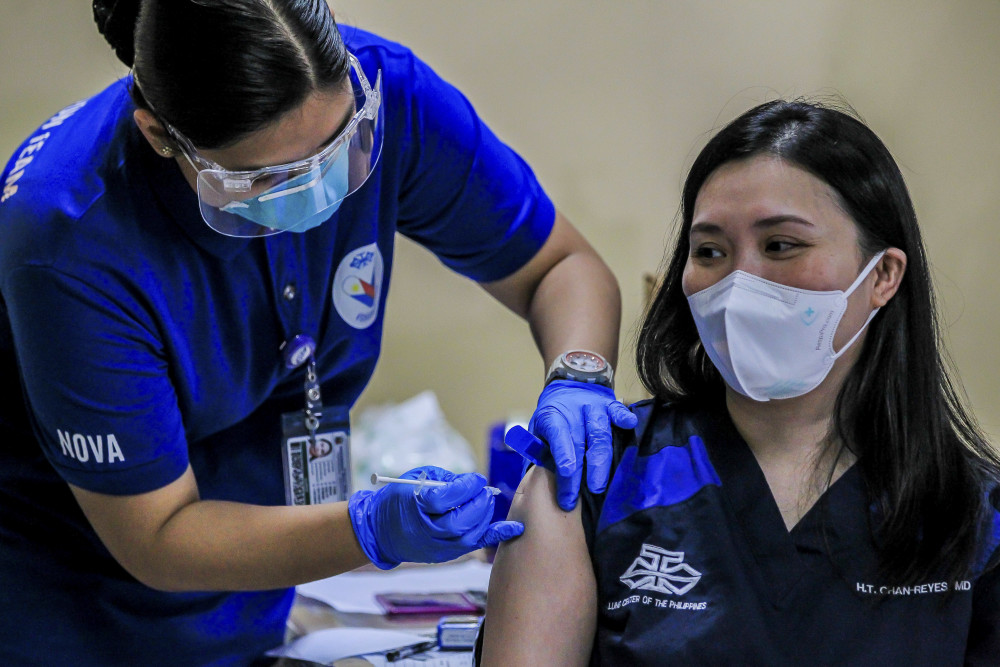
According to the Agencia EFE, vaccines developed by China’s Sinopharm, Sinovac, and CanSino Biologics were being put into use in Africa, Southeast Asia and Latin America. In Europe, Serbia and Hungary also used China’s vaccines. Guo Weimin, Spokesman for the Fourth Session of the 13th National People’s Congress, said that by the end of February 2021, China provided vaccine assistance to 69 countries and 2 international organizations and exported vaccines to 28 countries. The number was still increasing.
In the fight against the epidemic around the globe, the reliability, effectiveness and safety of vaccines developed by China are among the top ten in the world. Whether it is the anti-epidemic outcome or the vaccine research and development, China has contributed an indelible force to the global fight against the COVID-19, gaining valuable time for human to beat the virus.
The great performance of “the rising star”
For a long time, international vaccines are mainly developed by countries like the UK, the US, and France. China’s vaccine started relatively late, and there are still many new areas to be explored. However, in recent years, the launch of relevant laws and regulations on vaccine has provided a guarantee for its large-scale and standardized development and also laid a foundation for China’s vaccine to emerge on the world stage.
Therefore, we can see that China is always at the forefront of global vaccine research and development. The related enterprises have also made new breakthroughs in nucleic acid detection reagents and clinical therapy.
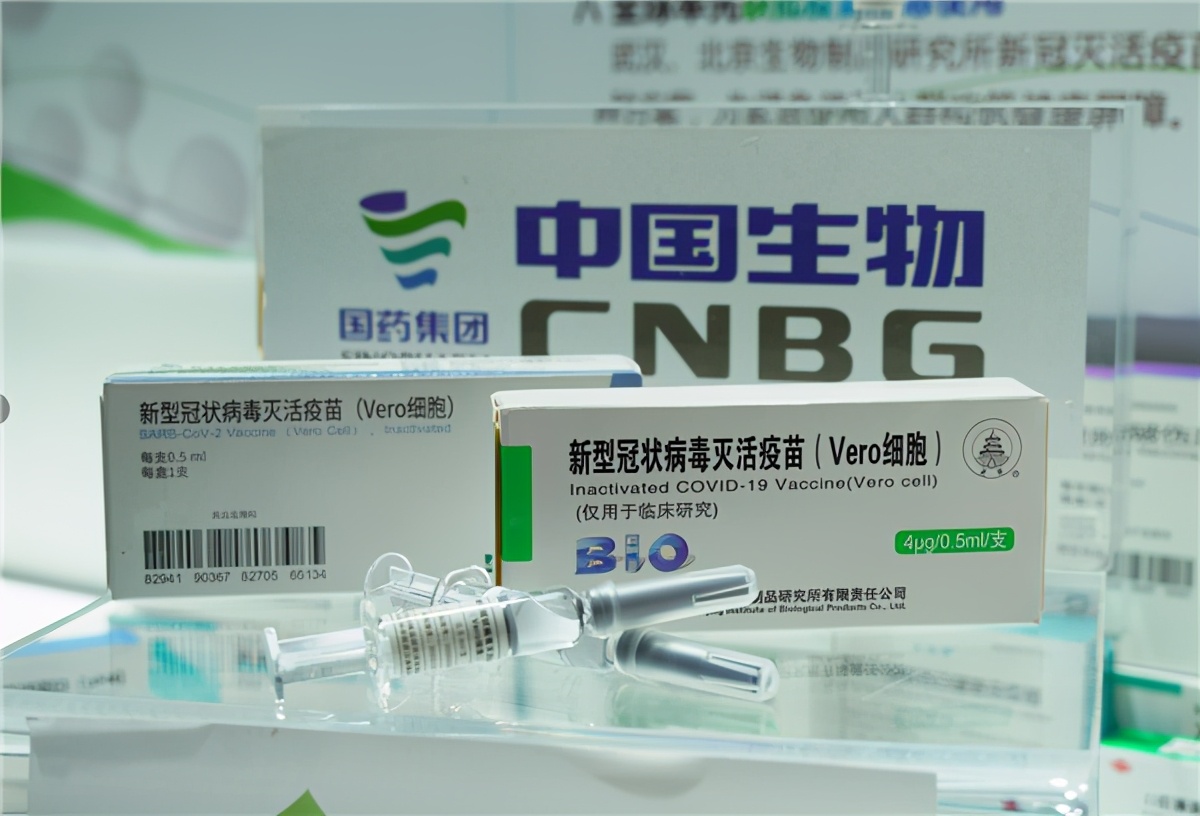
Wang Zhigang, Minister of Science and Technology of China, introduced on February 26, 2021 that five technical routes for vaccine research and development in China were carried out in parallel. Among the vaccines, 7 entered phase III clinical practice, and 4 were approved for listing but attached with conditions. 11 drugs or treatment methods entered the treatment plan. China participated in 10 WHO working groups, conducted exchanges and cooperation with the United States, countries in Europe, Asia, Africa and Latin America, the Caribbean and other countries in the fight against the epidemic, shared the latest achievements, and provided China approaches.
On March 4, 2021, Zhang Yesui, Spokesman for the Fourth Meeting of the 13th National People’s Congress, said that, so far, 17 vaccines have entered the clinical trial stage in China. In addition, China’s vaccine was also directly produced locally in cooperation with other countries through authorization. The method of “cooperative production” shortened the distance of vaccine transportation, further reduced the cost of transportation and storage, and facilitated the production and vaccination in other countries.
The future of China’s vaccine industry is promising
The impact of the epidemic can not be avoided, but to some extent, it also brings opportunities for the development of China’s pharmaceutical industry, including biological products and vaccines.
Since the epidemic, enterprises under the China Vaccine Industry Association have been actively involved in the fight against the epidemic. It is reported that in 2020, 13 enterprises under the China Vaccine Industry Association carried out vaccine research and development, and 8 of them entered the clinical trial stage.
Feng Duojia, President of the China Vaccine Industry Association, said that there are 18 vaccine production lines in China. By the end of 2021, the total production capacity of the vaccine in China is expected to exceed 2 billion doses, and it is likely to exceed 4 billion doses by the end of 2022.
He also said that excluding vaccine, the scale of China’s vaccine industry has reached RMB 35 billion yuan in 2020, with a total production capacity of 700 million doses, accounting for 16.4% of the global vaccine.
Industry insiders said that in 2021, the annual production capacity of China’s vaccine for COVID-19 will reach nearly three times of the previous national vaccine production capacity. Driven by the vaccine for COVID-19, the vaccine industry will welcome rapid growth, and the share of China’s vaccine in global market is expected to increase significantly. In addition, there is still a very broad space for self-paid vaccines, such as 4-valent influenza vaccine and hand, foot, and mouth disease vaccine.
China is undoubtedly a major vaccine producer. The level of its vaccine industry has also been among the world’s advanced ranks. But to become a powerful vaccine producer, China still has a long way to go. How can China’s vaccine industry go far and steadily in the future?

The core driving force of the development of the vaccine industry lies in technological innovation. As Peng Duojia said, represented by the COVID-19 vaccine, China’s vaccine is going global at a larger scale and faster speed. It is bound to have an impact on the traditional world vaccine market. Both supply and demand sides have to gradually understand and adapt to each other. It is both an opportunity and a challenge for China’s vaccine industry. The industry must meet international standards in terms of quality, conform to the international market in terms of service, and strengthen the awareness of serving global public health, so as to make the vaccine industry more concerned with the dignity of life as well as livelihood.
In the future, China’s vaccine industry needs to further strengthen the policy guidance of vaccine industry development, narrow the gap between China and developed countries in scientific and technological innovation, and meet the demand of vaccine manufacturers in some links of the industrial chain. With more efforts in these aspects, China’s pharmaceuticals can truly become global public products and inject more positive energy into global public health.
Source: China-ASEAN Panorama
桂ICP备14000177号 Copyright@2006-2013 Guangxi China-ASEAN Panorama Magazine Agency Co., Ltd. All Rights Reserved